- We believe that receiving high-quality services, appropriate after-sales support, and the preservation of our customers’ dignity and status are their fundamental rights. During the provision of services, our experts are always available to answer your questions. They strive to create a favorable experience for you by providing the necessary technical information for the optimal use of the equipment and its proper periodic maintenance.
Our Demanding Services
insurance for results
0+
number of servises
0+
our customers
0+
our team
0+
experinced years

- We believe that receiving high-quality services, providing appropriate after-sales support, and preserving the dignity and status of customers are their fundamental rights. During service delivery, our experts are always available to answer your questions and strive to create an optimal experience for you by providing the necessary technical information for better use of the equipment and more suitable periodic maintenance of the device.

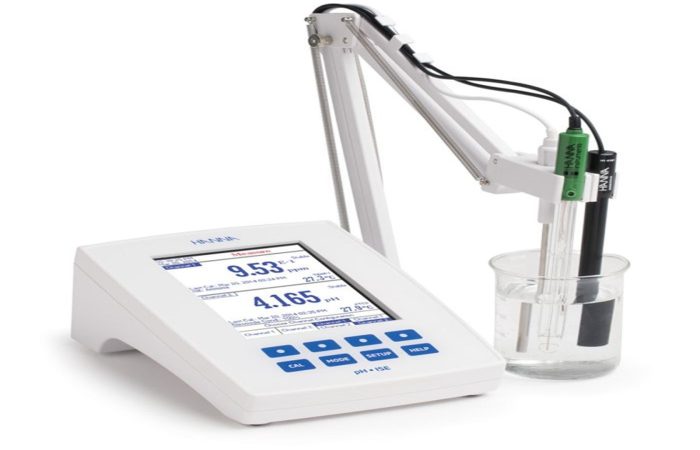
- Our experienced analysis team, with several years of expertise in pharmaceutical analysis, utilizes certified equipment and instruments alongside ISO 17025 and GLP quality systems to ensure a high level of confidence in the accuracy of results for you.

Reference materials, as part of the traceability chain, play an important role in chemical metrology. Given its comprehensive scope of services in the field of chemical measurement equipment metrology and instrumental analysis in Iran, as well as having a fully equipped analytical laboratory—and considering the access limitations and very high cost of these reference materials in the country—this company has undertaken the production of such reference materials.

Honor of cooperation with:






Our Certificates
Latest news and articles
You can follow the latest news and articles on laboratory equipment related to the pharmaceutical and healthcare industry through the links below.